Key Points:
- Nuclear fusion refers to what happens when two light atomic nuclei come together to create a single (much heavier) atomic nuclei that release an enormous amount of energy.
- Scientists hope to harness the power of nuclear fusion so that we as a society can finally begin to move toward true clean energy.
- Nuclear fission involves splicing elements into parts while fusion involves bringing two elements together. Fusion also involves heavy elements, while fission involves light ones.
©Andrey Suslov/Shutterstock.com
These days, with fossil fuels and radioactive waste posing serious threats to our earthly environment, there’s much talk of clean, renewable, sustainable energy. We see this in the increased number of solar panels on homes and businesses, the ever-growing number of electric cars sharing the road, and the continued spread of wind farms throughout our nation’s farmland. However, none of this is truly enough. Not when there’s the potential to harness the energy found in the sun and stars.
It sounds outlandish, but it’s totally attainable. In fact, Albert Einstein and his equation E=mc2 promised it. It’s called nuclear fusion, and, as you might have been able to surmise from the equation, it involves energy being created from the changing of an element’s mass. Unlike nuclear fission, where mass is reduced, fusion involves an increase in mass. It’s not as easy as installing an electrical outlet in the sun or in the stars, though. It’s not as easy as solving a simple equation, either. The key to nuclear fusion is a much harder one to crack. But what exactly is it? Is it even possible? And why does it matter?
Nuclear Fusion Explained
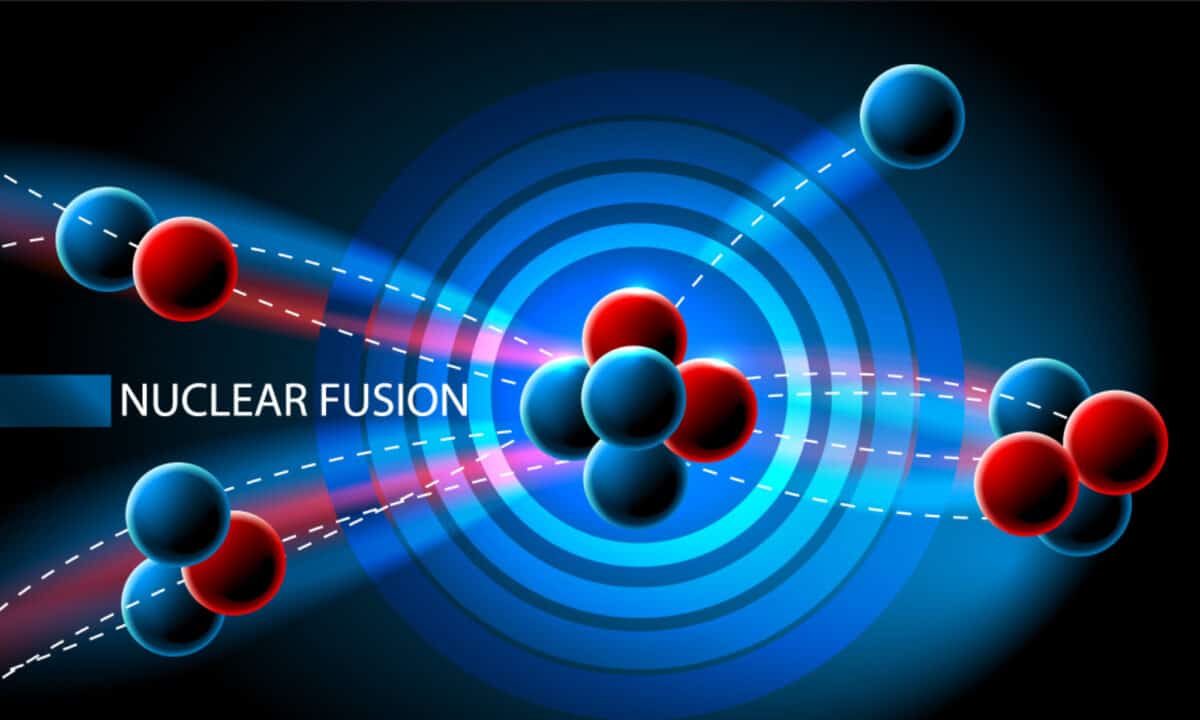
Nuclear fusion is the term used to describe what happens when two light atomic nuclei come together to create a single (much heavier) atomic nuclei that release an enormous amount of energy. It occurs in plasma, a state of matter distinct from the traditional three — solid, liquid, and gas. Instead, plasma is characterized by a high-temperature gas comprised of positive ions and free-moving electrons.
Nuclear fusion is more than just a concept. It exists in our natural world. As a matter of fact, the sun and the stars in space are all defined by nuclear fusion. For instance, the sun’s nuclei collide with one another at temperatures that exceed ten million degrees celsius. This gives the sun’s nuclei the energy they need to triumph over the inherent electrical repulsion of positively charged ions. At ten million degrees Celsius, the sun’s nuclei can begin to overpower that electrical aversion and begin to come together as one. (It also helps that the nuclei are trapped in a tiny space, which effectively raises the odds of them colliding.)
Naturally, nuclear fusion is a lot harder on Earth than it is on the sun. That’s because the sun is so much larger than our planet, which means the gravitational pull of the star keeps the necessary temperatures for nuclear fusion lower than here on Earth. Conversely, because we’re smaller than the sun, we need higher temperatures to achieve nuclear fusion. Therein lies the problem. Thankfully, scientists the world over are working tirelessly to resolve this problem.
Nuclear Fusion vs Nuclear Fission
©VectorMine/Shutterstock.com
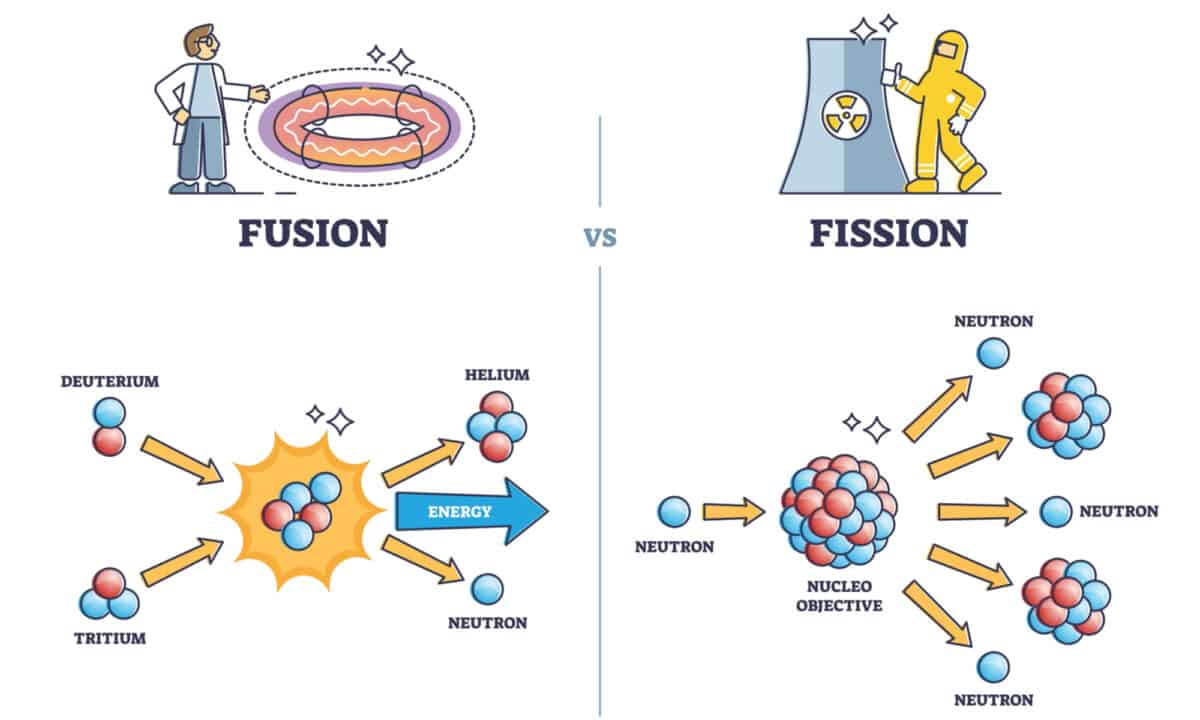
While nuclear fusion and nuclear fission sound more or less the same, there are a few key differences between the two that make them very distinct concepts. While fusion and fission are both forms of nuclear energy, fission involves splicing elements into parts while fusion involves bringing two elements together. Fusion also involves heavy elements, while fission involves light ones.
In both fusion and fission, large amounts of energy are released as nuclei go through their respective changes. Additionally, both reactions shift the reactant nuclei’s size in the direction of the higher bounded nuclei. However, the total amount of energy released differs between fusion and fission. Specifically, nuclear fusion generates much more energy than nuclear fission.
Another key difference is that fission generates unstable nuclei while fusion does not generate any lasting radioactive waste. The radioactive waste from fission can stay radioactive for millions upon millions of years, while fusion’s radioactive waste fades quickly thanks to its short half-life. It also helps that nuclear fusion produces more helium than radiation. (This is a totally safe gas that doesn’t pose any real threat.)
History of Nuclear Fusion
Research into the origins and the science of nuclear fusion first started in the early 20th century — the 1920s and ‘30s, to be more specific. Scientists wanted to know how and why the stars were able to shine. Before long, this evolved into a wider and more adventurous exploration of matter and energy on the whole. Looking beyond the stars, scientists began to see the big picture: whatever made those stars shine had the potential to completely reshape the way war, energy, transportation, and even space exploration were powered.
In 1932, John Cockcroft and Ernest Walton of the University of Cambridge were the first to use a particle accelerator for the purpose of nuclear experimentation. Before long, the boys had performed mankind’s first fission. To accomplish this man-made feat, Cockcroft and Walton took protons from the accelerator and used them to separate lithium into alpha particles. After this was done, they used the particle accelerator to fire deuterons at a slew of targets. This led to the very first man-made fusion of helions and tritons.
American and European experiments with nuclear fusion continued throughout the ‘30s, ‘40s, and well into the ‘50s. Japan, the Soviet Union, Sweden, and France also got in on the action, looking to find a new way to generate massive amounts of electricity. This widespread experimentation led to several other discoveries in the interim, such as lasers, thermonuclear weapons, and magnetic mirrors. Since then, from the 1960s through to today, both private and public research has continued at a rapid rate. Still, nobody seems to know exactly how to crack man-made nuclear fusion.
Because the sun and the stars are so much larger than the Earth, their gravitational pulls help facilitate nuclear fusion in ways we just can’t seem to do here on our planet. As such, scientists must bring hydrogen atoms to temperatures even higher than those on the sun — hundreds of millions of degrees Fahrenheit, at the very least. Researchers have tried every different kind of tool imaginable to get those temperatures up, but still no success. These have included everything from beams to microwaves to lasers. Rest assured, they’ll keep trying until they get it. The potential benefits are simply far too great to stop trying.

©42videography/Shutterstock.com
Advantages and Disadvantages of Nuclear Fusion
Advantages
- If successful, nuclear fusion plants would be much cheaper than our current sources of energy.
- The amount of energy that could be produced by nuclear fusion plants would far surpass the amount of energy produced by other means today.
- The process is very sustainable.
- It offers much less risk of environmental pollution.
Disadvantages
- It still hasn’t been accomplished by man.
- While it produces much less radioactive waste, there is still a production of radioactive waste.
- Once successfully accomplished, there’s a potential for nuclear fusion to be used to harm.
Is Nuclear Fusion the Future of Energy?
Scientists hope to harness the power of nuclear fusion so that we as a society can finally begin to move toward true clean energy. Truth be told, its reactions are four times more powerful than the reactions in nuclear fission. This means that nuclear fusion has a likely potential to become the future of energy in our species.
Plans are already being made for nuclear fusion reactors, though it’s uncertain how or when they’ll come to fruition. Scientists know that these nuclear fusion reactors will need to derive their energy from a combination of tritium and deuterium (which are two of the heaviest forms of hydrogen). This combo and the sheer amount of energy it could create if successful makes the mind reel. In theory, these planned nuclear fusion reactors have the potential to produce a whopping terajoule of energy with only a handful of grams of tritium and deuterium. (That’s equivalent to almost a lifetime of energy for a single person.)